Brief Introduction
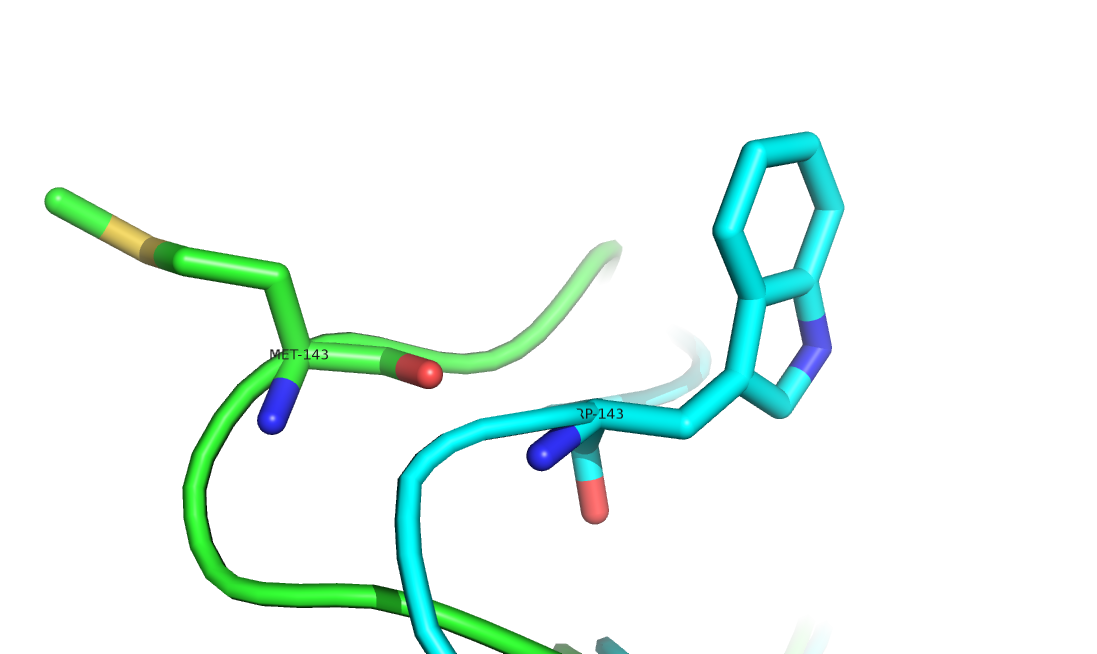
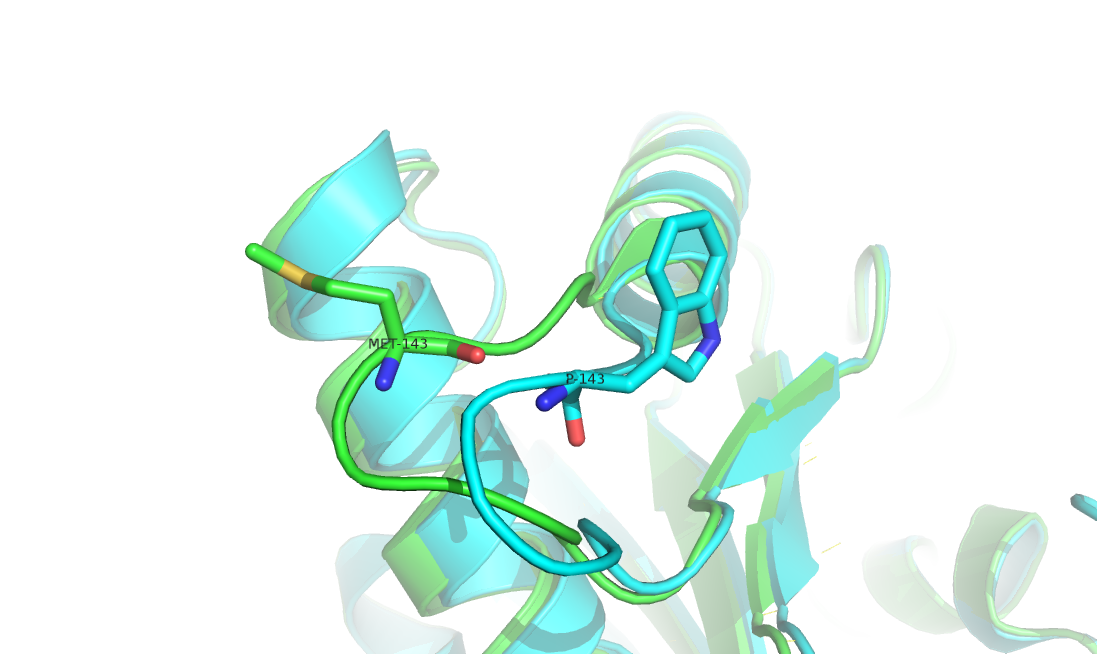
Substitutions of residues introduced by gene mutations can impact protein structures and often alternate protein properties. Identifying substitution-related structural changes is crucial for understanding their effects in biological functions. With the explosive increase of experimentally determined protein structures, collecting structures of residue substitution offers a great opportunity in studies of protein folding, design and engineering. In this work, we constructed a structure-pair database, named DRSP, which collected non-redundant pairs of proteins with single residue substitutions in the entire Protein Data Bank. It aims to (i) to facilitate analysis of protein structure and function changes induced by single residue difference, (ii) to deeper understand the mechanism of protein folding, and (iii) to infer possible rules for structure modeling and protein design. To our knowledge, DRSP is currently the largest publicly available database of the same kind. It provides abundant information such as substitution positions, types of amino acids, RMSDs, secondary structures, solvent accessible surface area, hydrogen bonds, and backbone/side-chain compatibility to illustrate structural changes that relate to residue substitution.
For more information click here.